+264 (0)61 240 140admin@n-c-e.org
Namibian Carnivore Working Group
Resources
Red Data book and poster
Conservation Status and Red List of the Terrestrial Carnivores of Namibia: Book

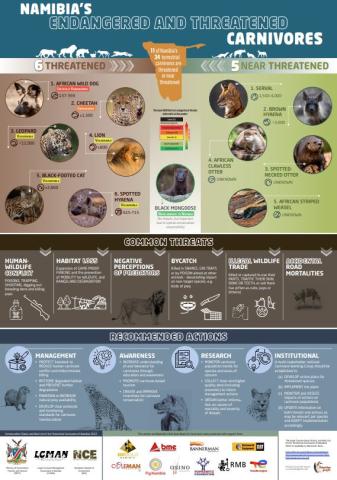
Conservation Status and Red List of the Terrestrial Carnivores of Namibia: Poster
Other resources
Castro-Prieto A et al. 2011. Cheetah paradigm revisited: MHC diversity in the world's largest free-ranging population. Molecular Biology and Evolution 28(4): 1455-1468. https://doi.org/10.1093/molbev/msq330
Castro-Prieto A et al. 2011. Diversity and evolutionary patterns of immune genes in free-ranging Namibian leopards (Panthera pardus pardus). Journal of Heredity 102(6): 653-665. https://doi.org/10.1093/jhered/esr097
Castro-Prieto A et al. 2012. Immunogenetic variation and differential pathogen exposure in free-ranging cheetahs across Namibian farmlands. PLoS ONE 7 (11): 1-19. https://doi.org/10.1890/ES12-00357.1
Costantini D et al. 2017. Socioecological and environmental predictors of physiological stress markers in a threatened feline species. Conservation Physiology 5(1). https://doi.org/10.1093/conphys/cox069
Durant SM et al. 2016. The global decline of cheetah Acinonyx jubatus and what it means for conservation. PNAS 114 (3): 528-533. https://doi.org/10.1073/pnas.1611122114
Edwards S et al. 2018. Coping with intrasexual behavioral differences: Capture– recapture abundance estimation of male cheetah. Ecology and Evolution 8(18): 9171-9180. https://doi.org/10.1002/ece3.4410
Edwards S et al. 2018. Making the most of by-catch data: Assessing the feasibility of utilising non-target camera trap data for occupancy modelling of a large felid. African Journal of Ecology 56(4): 885-894. https://doi.org/10.1111/AJE.12511
Edwards S et al. 2018. The spotted ghost: Density and distribution of serval Leptailurus serval in Namibia. African Journal of Ecology 56 (4): 831-840. https://doi.org/10.1111/aje.12540
Edwards S et al. 2019. Evidence of a high-density brown hyena population within an enclosed reserve: the role of fenced systems in conservation. Mammal Research 64: 519–527. https://doi.org/10.1007/s13364-019-00432-7
Edwards S et al. 2019. First confirmed record of infanticide for wild brown hyena. African Journal of Ecology 58 (3): 534-536. https://doi.org/10.1111/aje.12723
Edwards S et al. 2020. Socioecology of a high-density brown hyaena population within an enclosed reserve. Mammal Research 65: 223-233. https://doi.org/10.1007/s13364-020-00477-z
Edwards S et al. 2022. Cheetah marking sites are also used by other species for communication: evidence from photographic data in a comparative setup. Mammalian Biology https://doi.org/10.1007/s42991-022-00284-w
Weyrich A et al. 2022. First steps towards the development of epigenetic biomarkers in female cheetahs (Acinonyx jubatus). Life 12(6): 920. https://doi.org/10.3390/life12060920
Fischer H et al. 2022. A preliminary comparison of brown hyaena activity patterns at den sites located within a protected reserve and a commercial farmland. Namibian Journal of Environment 6B: 1-5
Frigerio D et al. 2018. Citizen science and wildlife biology: Synergies and challenges. Ethology 124 (6): 365-377. https://doi.org/10.1111/eth.12746
Giese L et al. 2021. Using machine learning for remote behaviour classification—verifying acceleration data to infer feeding events in free-ranging cheetahs. Sensors 21(16): 5426. https://doi.org/10.3390/s21165426
Government Gazette of the Republic of Namibia, No. 7912, 28 September 2022. Government Notice No. 278: Regulations relating to keeping of large carnivores in captivity: Nature Conservation Ordinance, 1975
Heinrich S et al. 2017. Cheetahs have a stronger constitutive innate immunity than leopards. Sci Rep 7. https://doi.org/10.1038/srep44837
Heinrich SK et al. 2016. Benign pigmented dermal basal cell tumor in a Namibian cheetah (Acinonyx jubatus). Case Reports in Veterinary Medicine. https://doi.org/10.1155/2016/7981765
Heinrich SK et al. 2016. Feliform carnivores have a distinguished constitutive innate immune response. Biology Open 5 (5): 550–555. https://doi.org/10.1242/bio.014902
Johnson S et al. 2013. Modeling the viability of the free-ranging cheetah population in Namibia: an object-oriented Bayesian network approach. Ecosphere 4(7): 1-19. https://doi.org/10.1890/ES12-00357.1
Kambongi T et al. 2021. A description of daytime resting sites used by brown hyaenas (Parahyaena brunnea) from a high-density, enclosed population in north-central Namibia. Namibian Journal of Environment 5B: 1-6
Klein K et al. 2021. Visual analytics of sensor movement data for cheetah behaviour analysis. Journal of Visualization 24: 807–825. https://doi.org/10.1007/s12650-021-00742-6
Krengel A et al. 2015. Gammaretrovirus-specific antibodies in free-ranging and captive Namibian cheetahs. Clinical and Vaccine Immunology 22(6): 611-617. https://doi.org/10.1128/CVI.00705-14
Krücken J et al. 2021. Genetic diversity of vector-borne pathogens in spotted and brown hyenas from Namibia and Tanzania relates to ecological conditions rather than host taxonomy. Parasites Vectors 14: 328. https://doi.org/10.1186/s13071-021-04835-x
Melzheimer J et al. 2018. Queuing, takeovers, and becoming a fat cat: Long-term data reveal two distinct male spatial tactics at different life-history stages in Namibian cheetahs. Ecosphere 9 (6). https://doi.org/10.1002/ecs2.2308
Melzheimer J et al. 2022. Communication hubs of an asocial cat are the source of a human–carnivore conflict and key to its solution. Proceedings of the National Academy of Sciences 117 (52). https://doi.org/10.1073/pnas.2002487117
Menke S et al. 2022. Oligotyping reveals differences between gut microbiomes of free-ranging sympatric Namibian carnivores (Acinonyx jubatus, Canis mesomelas) on a bacterial species-like level. Frontiers in Microbiology 5. https://doi.org/10.3389/fmicb.2014.00526
Menke S et al. 2017. Effects of host traits and land-use changes on the gut microbiota of the Namibian black-backed jackal (Canis mesomelas). FEMS Microbiology Ecology 93(11). https://doi.org/10.1093/femsec/fix123
NCE Press Release, 12 September 2022: Publication of Conservation Status and Red List of the Terrestrial Carnivores of Namibia
Noack J et al. 2019. Leopard density estimation within an enclosed reserve, Namibia using spatially explicit capture-recapture models. Animals 2019 (9): 724. https://doi.org/10.3390/ani9100724
Paijmans JLA et al. 2021. African and Asian leopards are highly differentiated at the genomic level. Current Biology 31(9): 1872-1882. https://doi.org/10.1016/j.cub.2021.03.084
Portas R et al. 2021. GPS telemetry reveals a zebra with anthrax as putative cause of death for three cheetahs in the Namib Desert. Frontiers in Veterinary Science 8. https://doi.org/10.3389/fvets.2021.714758
Portas R et al. 2022. Leopard Panthera pardus camera trap surveys in the arid environments of northern Namibia. Mammalian Biology. https://doi.org/10.1007/s42991-022-00237-3
Richmond-Coggan L. 2019. The Namibian leopard: National census and sustainable hunting practices. Report for Namibian Professional Hunting Association (NAPHA) and the Ministry of Environment and Tourism (MET)
Schares G et al. 2021. Molecular analysis suggests that Namibian cheetahs (Acinonyx jubatus) are definitive hosts of a so far undescribed Besnoitia species. Parasites Vectors 14: 201. https://doi.org/10.1186/s13071-021-04697-3
Seltman A et al. 2020. Species-specific diferences in Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti seroprevalence in Namibian wildlife. Parasites Vectors 13: 7. https://doi.org/10.1186/s13071-019-3871-3
Stein A et al. 2011. Namibian national leopard survey
Stein AB et al. 2011. Leopard population and home range estimates in north-central Namibia. Journal of Ecology 49 (3): 383-387. https://doi.org/10.1111/j.1365-2028.2011.01267.x
Thalwitzer S et al. 2010. Seroprevalences to viral pathogens in free-ranging and captive cheetahs (Acinonyx jubatus) on Namibian farmland. Clinical and Vaccine Immunology 17(2): 232-238. https://doi.org/10.1128/CVI.00345-09
Tordiffe ASW et al. 2016. Comparative serum fatty acid profiles of captive and free-ranging cheetahs (Acinonyx jubatus) in Namibia. PLoS ONE 12. https://doi.org/10.1371/journal.pone.0167608
Turner WC et al. 2022. Africa's drylands in a changing world: Challenges for wildlife conservation under climate and land-use changes in the Greater Etosha Landscape. Global Ecology and Conservation 38: 1-24. https://doi.org/10.1016/j.gecco.2022.e02221
Voigt CC et al. 2013. A breath test to assign carnivore diets to browsers or grazers. Wildlife Biology 19(3): 311-316. https://doi.org/10.2981/13-012
Voigt CC et al. 2018. Sex-specific dietary specialization in a terrestrial apex predator, the leopard, revealed by stable isotope analysis. Journal of Zoology 306(1): 1-7. https://doi.org/10.1111/jzo.12566
Voigt CC et al. 2014. The conflict between cheetahs and humans on Namibian farmland elucidated by stable isotope diet analysis. PLOS ONE 9(8): e101917. https://doi.org/10.1371/journal.pone.0101917
Wachter B et al. 2010. Reproductive history and absence of predators are important determinants of reproductive fitness: the cheetah controversy revisited. Conservation Letters 4: 47-54. https://doi.org/10.1111/j.1755-263X.2010.00142.x
Wachter B et al. 2012. An advanced method to assess the diet of free-ranging large carnivores based on scats. PLoS ONE 7(6): e38066. https://doi.org/10.1371/journal.pone.0038066
Wasimuddin et al. 2017. Gut microbiomes of free-ranging and captive Namibian cheetahs: Diversity, putative functions and occurrence of potential pathogens. Molecular Ecology 26(20): 5515-5527. https://doi.org/10.1111/mec.14278
Weise FJ et al. 2017. The distribution and numbers of cheetah (Acinonyx jubatus) in southern Africa. PeerJ 5:e4096. https://doi.org/10.7717/peerj.4096





